Stanford Begins Trials With Pfizer on Children Under Age 5
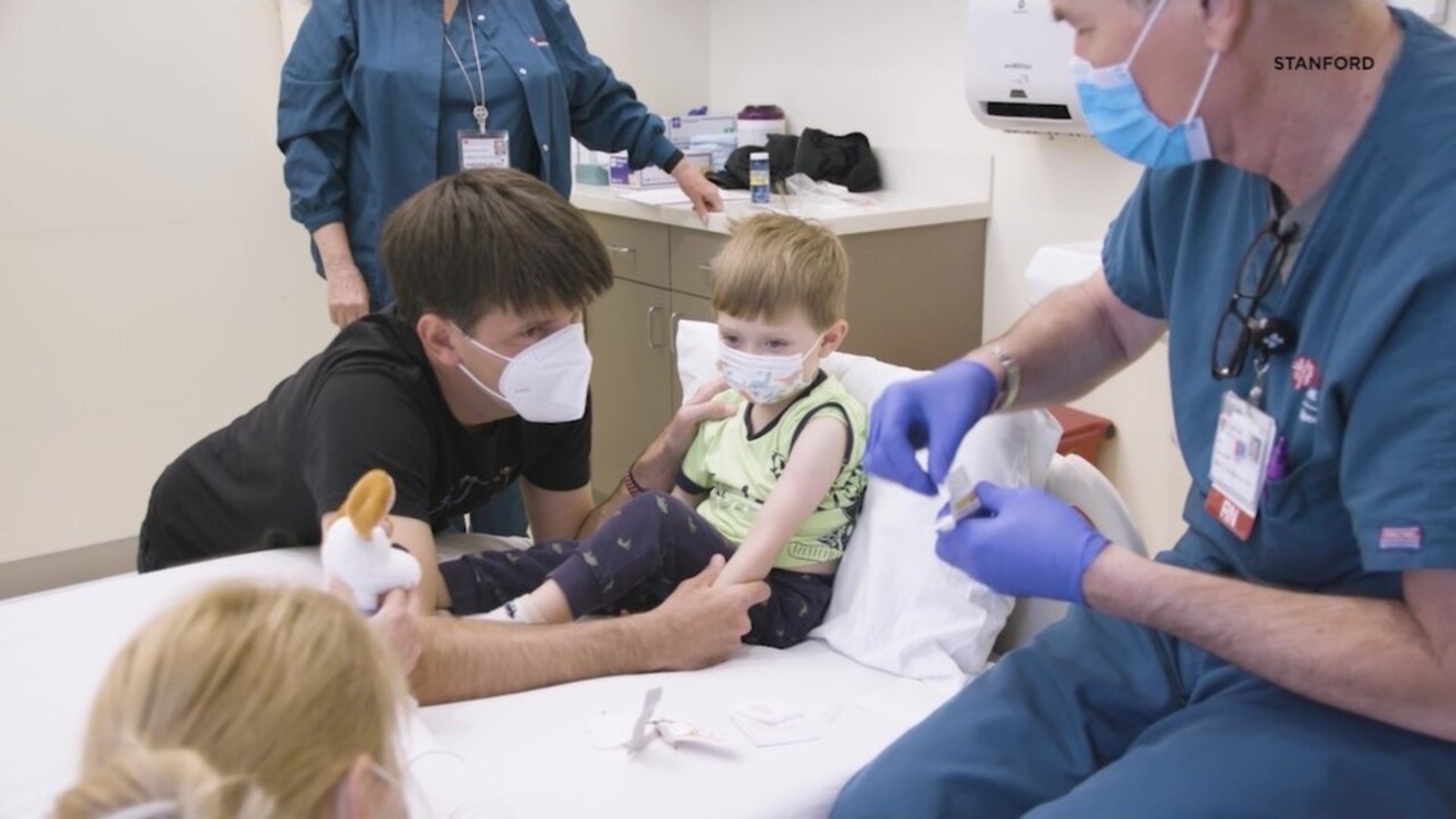
The next critical step in reaching herd immunity is being able to vaccinate children and Stanford announced Wednesday the process is already underway.
Stanford is part of a trial with Pfizer to test the COVID-19 vaccine on children under the age of 5, and 3-year-old Eloise Lacour is an important part of this anticipated trial.
“We know that getting herd immunity is a big part of getting back to whatever normal will look like in the future,” said Eloise’s parents Angelica and Chris Lacour.
“We were there for the entire process, they walked us through, made sure all our questions were answered, we sat with her,” they said as they explained the process.
Stanford is only one out of five sites nationwide participating in the trial which goal is to ensure that children can safely take and tolerate the vaccine.
Children’s reaction to the vaccine is a critical factor to consider given millions of children in the country have been infected with the virus.
“Children shouldn’t be getting sick,” said Dr. Yvonne Maldonado, Stanford’s Infectious Disease Specialist. “This is a devastating issue to deal with, and we don’t know what long-term issues there may be with infected children.”
Eloise parents say they’re happy to be part of this trial and are proud of their daughter for being able to participate.

